Basel, Switzerland, January 22, 2025 — The Mario Negri Institute for Pharmacological Research...
Revolutionizing CAR-T cell production: Magnetic bead-sensitized optoporation for mRNA delivery
Basel, Switzerland, March 13, 2025 — Scientists demonstrate successful CAR-T cell engineering using optoporation, a non-viral gene delivery technique that improves cell viability and potentially reduces manufacturing costs. Researchers employed CATUG CD19 CAR mRNA as a proof of principle, evaluating its efficiency and functional activity against electroporation.
Immunotherapy is rapidly advancing, with engineered T cells showing significant promise in the treatment of hematological malignancies. However, the current standard of using viral vectors for genetic modification poses substantial regulatory, cost, and safety hurdles.
Researchers from the University of Geneva, the University Hospital of Geneva, the Swiss Cancer Center Léman, Agora Cancer Research Center, and Limula SA have joined forces to publish innovative research. Noelia Maldonado-Pérez, Marie-Agnès Doucey, Dzhangar Dzhumashev, Darel Martínez Bedoya, Luis Castillo Cantero, Caroline Boudousquie, Yann Pierson, Luc Henry, Valérie Dutoit, and Denis Migliorini introduce a groundbreaking alternative: magnetic bead-sensitized optoporation, a novel approach for non-viral gene delivery in CAR-T cell manufacturing published this March 2025 in Molecular Therapy: Methods & Clinical Development.





A paradigm shift in non-viral mRNA delivery
Traditionally, electroporation uses electric pulses to create temporary pores in the cell membrane and it has been the primary method for mRNA transfection in T cells. While effective, electroporation can induce significant cytotoxicity and variability in gene expression. In contrast, optoporation uses pulsed laser energy to transiently permeabilize cell membranes, facilitating efficient mRNA delivery without compromising cell viability.
CD3/CD28 magnetic beads are superparamagnetic beads coated with antibodies targeting CD3 and CD28, two key receptors involved in T cell activation. These beads are widely used in immunotherapy to activate and expand T cells in vitro. In this study, they serve a dual purpose:
-
T Cell activation:
- CD3/CD28 beads mimic antigen-presenting cells by stimulating the T cell receptor (CD3) and costimulatory receptor (CD28).
- This stimulation helps drive T cell proliferation and prepares them for genetic modification.
-
Enhancing mRNA delivery:
- In addition to activating T cells, these beads also act as photosensitizers during optoporation.
- When exposed to pulsed laser energy, the beads absorb the light and induce local membrane permeabilization, allowing mRNA to enter the cells more efficiently.
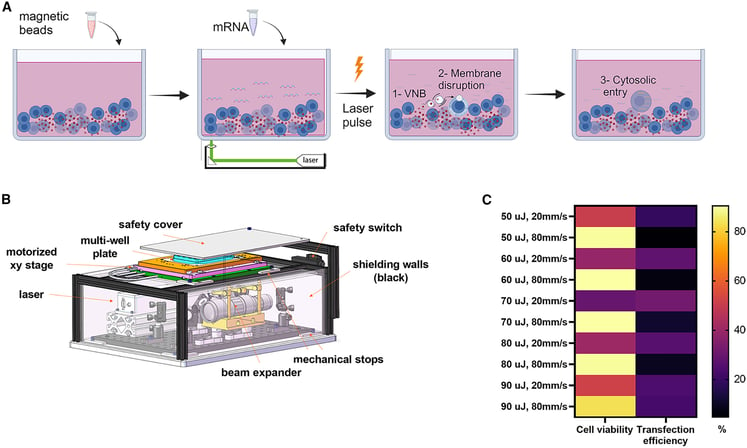
CATUG is proud to have provided the CD19 CAR mRNA used in this study as proof of principle, to test optoporation transfection efficiency and functional activity compared to electroporation, contributing to a transformative technology that enhances the development of next-generation CAR-T cell therapies.
Notably, T cells optoporated with CD19 mRNA exhibited high functionality and proliferation capacity, surpassing electroporated counterparts in key performance metrics. The study demonstrates that magnetic bead-sensitized optoporation enables the successful transfection of primary human T cells with mRNA encoding CAR constructs.
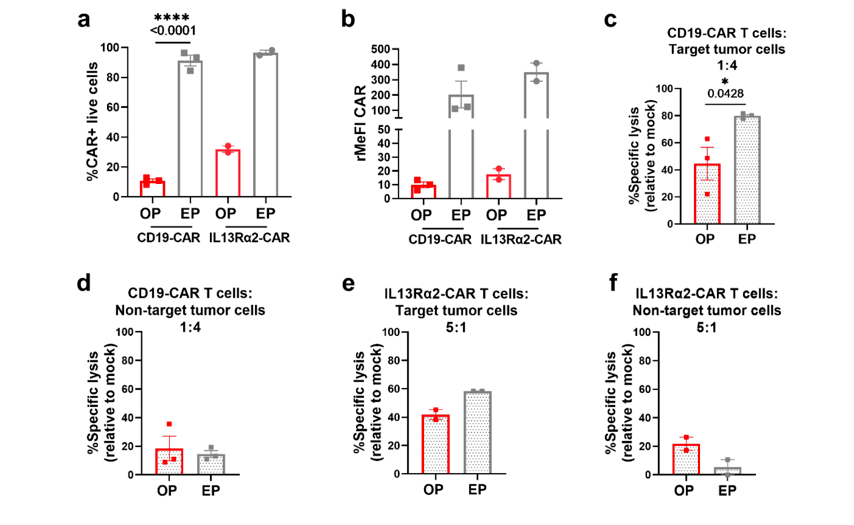
This methodology simplifies the workflow and improves transfection efficiency. Additionally, flow cytometry analysis confirmed that optoporated T cells maintained a comparable memory and activation profile to electroporated cells, with preserved cytokine secretion. Notably, optoporated T cells exhibited superior proliferation, indicating potential long-term therapeutic benefits.
Advantages of magnetic bead-sensitized optoporation
1. Enhanced Cell Viability: Unlike electroporation, which can induce high levels of apoptosis, optoporation preserves cell integrity and function, leading to superior expansion potential.
2. Scalability & Cost-Effectiveness: By eliminating the need for proprietary electroporation buffers and complex viral vector manufacturing, this method reduces production costs and enhances accessibility for clinical applications.
3. Regulatory Benefits: The non-integrative nature of mRNA delivery eliminates concerns regarding insertional mutagenesis, addressing safety issues that have been raised in recent reports of secondary malignancies associated with viral CAR-T therapies.
4. Versatility in Immune Cell Engineering: The technique has demonstrated efficacy not only in T cells but also in natural killer (NK) cells and monocytic cell lines, broadening its potential applications in cell therapy.
Towards the future of mRNA-based immunotherapies
We extend our sincerest congratulations to the authors for their exceptional work. The integration of magnetic bead-sensitized optoporation into CAR-T cell manufacturing represents a significant step forward in the search for safer, more efficient, and cost-effective immunotherapies. As the industry shifts towards non-viral methodologies, we are excited to be at the forefront of this transition, providing high-quality mRNA solutions for transformative cell-based treatments.


