mRNA-LNP CDMO
We aim to help our clients advance to the next stage by providing all possible solutions during the complete life cycle of drug development.
Our mRNA & LNP Services
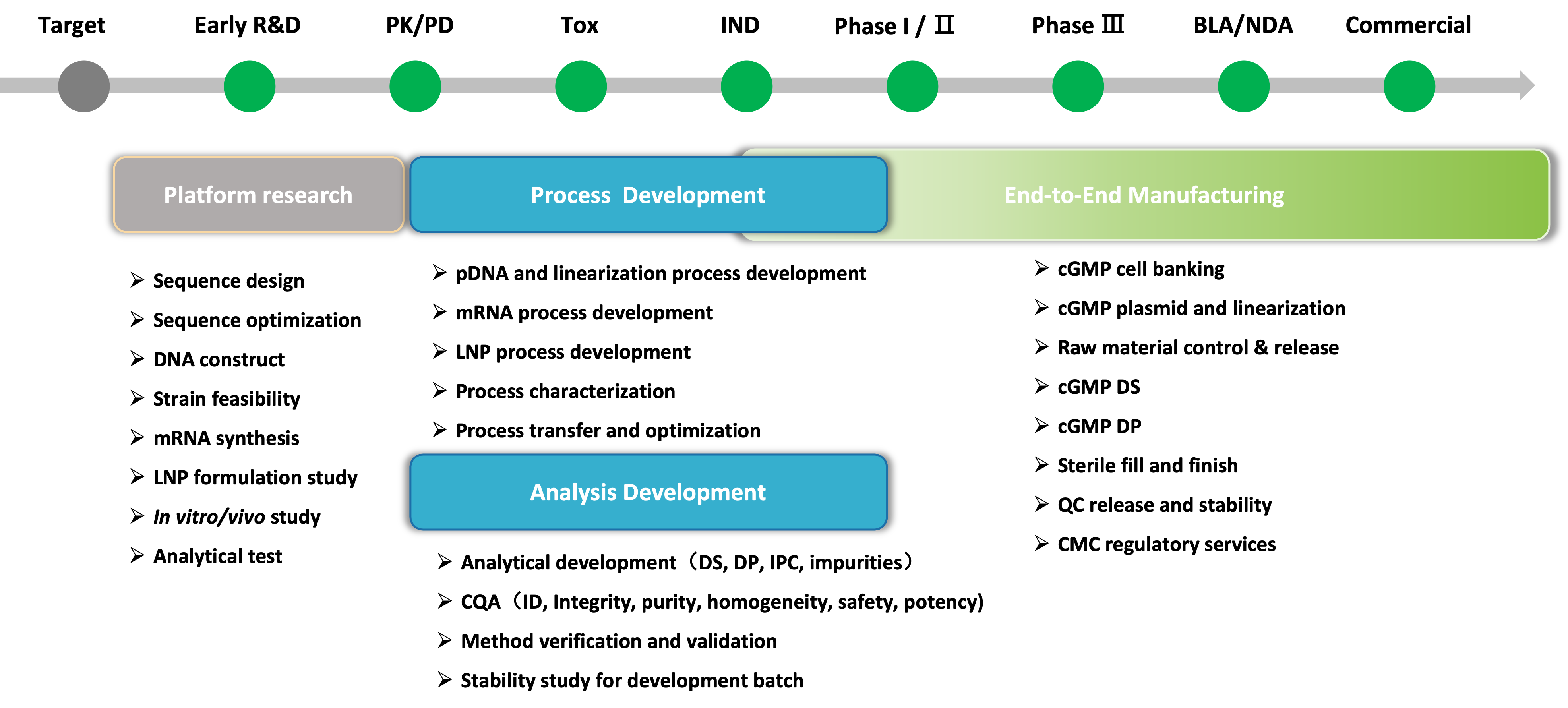
CATUG mRNA Platform

Flexible Scale
From 100 mg up to 100 g scale mRNA synthesis for use from R&D to pre-GMP and GMP
High Integrity
Strong know-how on mRNA process to produce high integrity high purity mRNA drug substance
High Yield
Efficient and stable process to achieve high IVT yield upstream and high recovery rate downstream

Low Immunogenicity
CATUG in-house technology for dsRNA removal could dramatically reduce the immunogenicity of mRNA therapeutics

High Throughput
In-house high throughput IVT synthesis and purification for early R&D mRNA screening
Analytical Support
Over 50 in-house analytical methods for mRNA DS and DP release testing and quality research
CATUG LNP Platform

Max MixTM
CATUG in-house process and equipment for large-scale (up to 100g) mRNA encapsulation process

Microfluidic
Collaboration with PNI on microfluidic mixing process supporting from early R&D to cGMP LNP production
Flexible Scale
From 1 mg up to 100 g mRNA encapsulation for use from R&D to pre-GMP and GMP
Formulation Study
Strong expertise in LNP formulation study and in vitro/vivo screening for early R&D
Customized DoE
Provide customized DoE studies during process development for better mixing outcomes and stable products
Analytical Support
Over 50 in-house analytical methods for mRNA DS and DP release testing and quality research
